Polymers are large molecules called macromolecules, which are usually organic and are formed by the union of smaller molecules called monomers, forming huge chains of the most diverse shapes.
What is a synthetic polymer?
There are several types of polymers with different properties and chemical structures. Synthetic polymers are those obtained in laboratories or in industry. Some examples of synthetic polymers are nylon, polystyrene, polyvinyl chloride (PVC), polyethylene, etc.
Synthetic polymers are created by man from elements found in nature. These synthetic polymers are created for specific functions and have the characteristics to fulfill these functions.
Structure of a polymer
A polymer is made up of molecules (the basic unit that forms a chemical compound), called monomers, often linked together to form a linear chain. Each molecule can be of natural or synthetic origin, and have a low molecular weight (MW). This magnitude is the ratio between the average mass of a substance, per molecule of its specific isotopic composition, and 1/12th of the mass of the carbon-12 atom.
The bonding between molecules occurs through chemical reactions (Figure 1). The number of monomers bonded can be in the hundreds or thousands, bringing the molecular weight of the polymer to values in the range of 1,000 to 1,000,000. This number n is the degree of polymerization (DP).
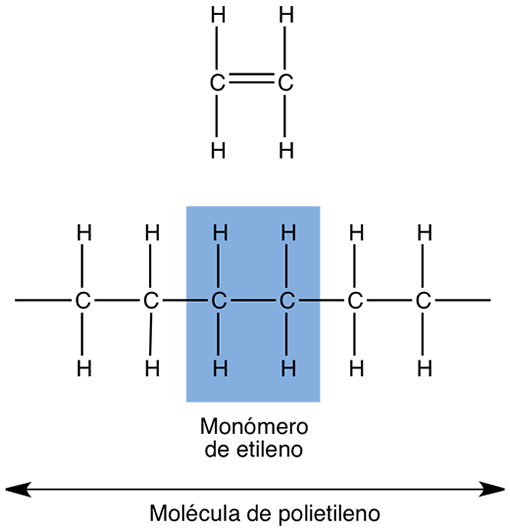
Figure 1: Formation of the polymer polyethylene (a plastic) from the union of several ethylene monomers (a), promoted by the conversion of the C = C double bond into two C – C single bonds.
A typical example of a synthetic polymer is the one formed from the monomer ethylene, which by reaction with molecules of the same type forms polyethylene, or simply, PE (Figure 1). The chemical reaction for the synthesis of the polymer is called polymerization. The ethylene molecule is CH 2 =CH 2 and joins with n other molecules to form the polymer (Figure 2).
The main characteristic of polymers is their high molecular weight, which determines the chemical and physical properties of these molecules. As polymerization progresses, the degree of polymerization increases, as does the molecular weight of the polymer. There are also naturally occurring polymers produced by organisms: polysaccharides (cellulose and starch), proteins (collagen, hemoglobin, hormones, albumin, etc.) and nucleic acids (DNA and RNA). Both polymers and these molecules are classified as macromolecules.
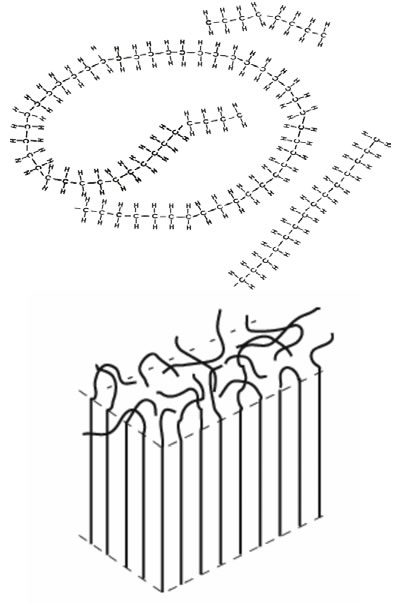
Figure 2: Two-dimensional (top) and three-dimensional representation of the “spaghetti”-like structure of solid polyethylene.
Importance of synthetic polymers
The objects that we use most frequently and on a daily basis are synthetic polymers and rubbers.
Synthetic polymers are widely used in the manufacture of packaging for food products, drugs and chemicals, household appliances, tools, household utensils, toys, and automotive components; this is part of a very long list of applications. Polymers are also used in various areas of science and technology.

This widespread use is due to the low production cost, low density, adequate toughness, good surface finish, durability, versatility of the production system, among other advantages over metallic or ceramic materials. Also, it is necessary to note that many products originally made with other materials were replaced by objects designed in plastic materials. Thus, for example, automotive components such as chrome-plated metal bumpers have been replaced by others made of reinforced plastic due to the lower production cost and greater resistance to corrosion after suffering impacts. Other substitute parts have been the dashboard, the steering wheel, the internal roof lining, the upholstery and padding of the seats, components of the safety belts, the covering of electrical cables, hoses, containers for liquids and the joints, etc. In addition, this intensive use of plastic and rubber allows the reduction of the weight of the car, lower fuel consumption, greater comfort and safety for the passenger.

The advantages mentioned are counterbalanced by the slow degradation of these materials, which can last for several years, generating significant environmental problems. Recycling these materials is even more expensive than producing them from virgin materials.
Synthetic polymers are mainly derived from petroleum (a mixture of hydrocarbons). 4% of the world’s oil production is converted into polymers. After a cracking and reforming process, simple molecules such as ethylene, benzene, etc. are obtained, from which the polymer synthesis will begin.
The synthesis of any polymer, with controlled quality, is a very complex process, and we will normally find the large petrochemical industry associated with the production of various plastic materials. This industry will supply finished material susceptible to subsequent moulding, in the case of thermoplastics, or reactive liquids and powders, in the case of thermosets, lacking the final cross-linking reaction.