The electron is one of the three main particles that make up the atom . The other two particles in the nucleus of an atom are protons and neutrons. Unlike protons and neutrons, which are composed of simpler particles, electrons are “fundamental particles” that do not include smaller particles. In fact, an electron is a fundamental particle called a ” lepton “. All leptons have an electric charge of−1until the0have. In a solid , the main means of current transfer are electrons, and this concept is also expressed with the help of metallic bonds . In liquids too, this current is caused by ions .
Table of contents of this article
Discover the electron
At the end of the 19th century, an English physicist named “Sir Joseph John Thomson” conducted an experiment with a cathode ray tube. A cathode ray tube is a glass tube with most of its air evacuated.
By applying a high voltage to the electrodes at one end of the tube, a beam of particles flowed from the cathode to the anode. These rays can be observed using a colored substance containing phosphorus .
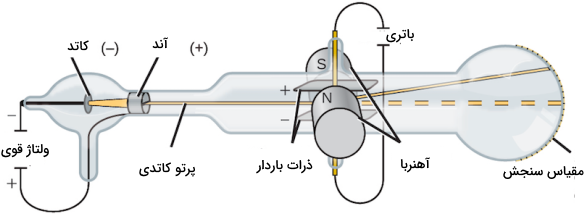
In order to investigate the properties of particles, Thomson placed two electrically charged plates with an inhomogeneous charge around the cathode ray tube. When the cathode ray passes, it is repelled from the negatively charged plate and absorbed towards the positively charged plate. This experiment showed that the cathode ray is composed of negatively charged particles.
Additionally, Thomson placed two magnets around the cathode ray tube and observed that the magnetic field also deflected the cathode ray. As a result of these experiments, Thomson succeeded in measuring the “mass-to-charge” ratio of cathode ray particles. With the help of this experiment, it was found that the mass of each particle is much less than any known type of atom.
Thomson also performed this experiment with other metal electrodes and found that the properties of the cathode ray remained constant and were therefore independent of the material of the electrode used. Thomson presented the following results with the help of his experiment findings:
- Cathode rays are made up of negatively charged particles.
- These particles must be part of an atom because the mass of each particle is only12000The mass of the hydrogen atom is
- These subatomic particles exist in all atoms.
At first, Thomson’s results had opponents, but over time they were accepted by scientists. Finally, the cathode ray particles of Thomson’s experiment were given a name, which was electrons. The discovery of the electron violated part of Dalton’s atomic theory, which stated that atoms cannot be divided into smaller particles. In order to describe the presence of electrons, a newer atomic model was needed.
Thomson raisin cake model
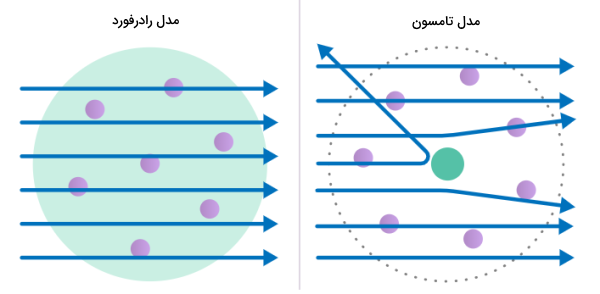
Thomson knew that atoms are electrically neutral. Consequently, he postulated that there must be a source of positive charge in the atoms to neutralize the negative charge of the electrons. This led Thomson to state that atoms can be described as negatively charged particles moving among positive charges. This model is commonly known as “Plum Pudding Model”.
According to our current knowledge about the atom, we know that Thomson’s raisin cake model is not a correct model. Scientists continued to investigate the structure of atoms and even evaluated the accuracy of Thomson’s atomic model.
Ernest Rutherford and the gold leaf experiment
The next experiment that was conducted regarding the structure of the atom was the experiment conducted by the New Zealand scientist, Ernest Rutherford . In this experiment, Rutherford shined a narrow beam of alpha particles on a thin sheet of gold . For this purpose, Rutherford placed a sample of radioactive metal called radium inside a lead box that had a small opening to the outside. Most of the rays were absorbed by the lead box, but a beam of alpha particles passed through the aperture and hit the gold foil. The gold foil was also surrounded by a detector that revealed the presence of the alpha beam.
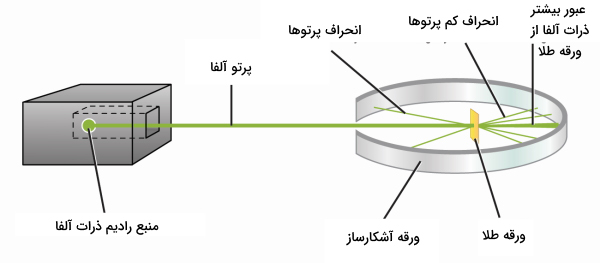
Based on Thomson’s raisin cake model, it was assumed that the positive charges are scattered in the volume of the atom and as a result, most of the alpha particles should easily pass through the gold foil because the strength of the magnetic field resulting from the positive particles is very weak and has an effect. It will not have alpha on the path of heavy and fast particles.
The results of the experiment were different from the expected results. While all the alpha particles passed straight through the gold foil, a few were deflected by more than 90 degrees . Ruderfoder described the results of his experiment as follows:
“This experiment was the strangest event that has ever happened in my life. The wonder of it is as if you shot a 15-inch ball and it hit you by hitting the thin gold sheet and changing its trajectory.
Nuclear model of the atom
Based on his experimental findings, Rutherford provided the following conclusions for the structure of an atom:
- The positive charge must be in a very small volume of the atom, which makes up most of the mass of the atom. This explains why a small fraction of the alpha particles deviated from their path by a large margin, and this deviation may be due to collision with the gold nucleus.
- Since most of the alpha particles passed directly through the gold foil, as a result, most of the space of the atom is empty volume.
All these things caused Rutherford to present his nuclear model. In this model, the atom consisted of a very small positive nucleus surrounded by negative electrons. Based on the number of alpha particles deflected in Rutherford’s experiment, Rutherford calculated that the nucleus of an atom makes up a small fraction of its volume.
Rutherford’s nuclear model (atomic model) described his laboratory results but brought other questions and ambiguities. For example, the question was asked what is the role of electrons in the empty space of the atom. Next, Niels Bohr proposed new experiments, with the help of which, finally, a new model of quantum mechanics was presented.
Electron and antiparticle
symbol�are considered to represent an electron. The antiparticle of the electron, which carries a positive charge, is called a “positron” or antielectron, and it is called�+They show. It should be noted that due to the collision of an electron and a positron, gamma rays are released in an explosive process.
The interaction of the electron with other subatomic particles forms a branch of chemistry called nuclear chemistry.
Electron properties
Electrons are very small particles. The mass of an electron is approx12000Mass is a proton or neutron. Therefore, electrons do not contribute to the mass of an atom. Electron electric charge equal to−1has an equal but opposite electric charge+1is in the proton. Neutral atoms all have an equal number of electrons and protons, and as a result, these positive and negative charges cancel each other out.
The location of electrons
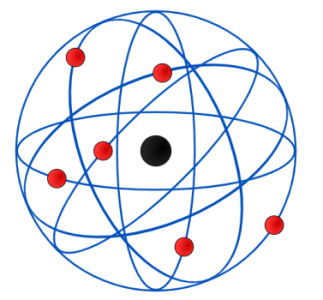
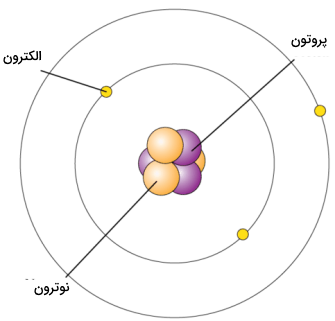
Unlike neutrons and protons, which are located inside and at the center of the atom’s nucleus, electrons must be found outside the nucleus. Since opposite charges attract each other, the negative electron is attracted to the positive nucleus. This attraction will cause the electrons to move in the empty space around the nucleus. The image below is usually used to show the structure of an atom. This image shows the electron as a particle orbiting the nucleus. The movement of electrons around the nucleus has complexities that are explained by quantum mechanics.
Orbitals
The atomic model in the previous picture is a suitable model, but it does not have the necessary efficiency when displaying the location of the electron in the atom. In fact, the path of an electron cannot be determined and only the probability of the presence of the electron in a certain area around the nucleus can be determined. The range in which the electron is most likely to be present is known as ” Orbital “. Each orbital can eventually hold up to two electrons. Orbitals also have shape. s-type orbitals have a spherical shape and p-type orbitals have a shape like a dumbbell. In the picture below, you can see the shape of these two orbitals. Places with a higher density of points indicate a higher probability of electron presence in those areas.

Energy levels
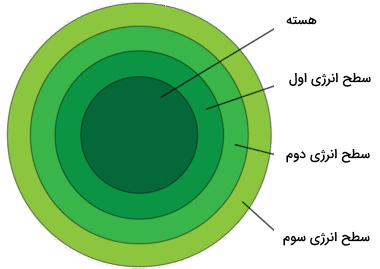
Electrons are located at a fixed distance from the nucleus, and these fixed distances are called energy levels. In the next picture, you can see the three basic levels of energy. This image also shows the maximum number of electrons in each level.
Tips on energy levels
In the following, we intend to express more points about energy levels.
- Electrons in lower energy levels have less energy and are closer to the nucleus. In the lowest energy level, there is only one orbital, so this energy level will have only two electrons.
- Electrons move to a higher energy level only when the lower energy level is filled. Electrons in higher energy levels have more energy. These levels will also have more orbitals and therefore more electrons.
- The electron that is in the outermost energy level of an atom is called valence electron or valence shell electron. This type of electrons determine many properties of an element because these electrons are involved in many chemical reactions with other atoms. Atoms may share their valence electrons or exchange them in bonds .
Facts about electrons
In the following, we will discuss various things about an electron.
Electron radius
For electrons, radius and therefore volume are not considered. In fact, electrons are a type of “point particle” that also has a wave-like behavior.
Electrons and compounds
As mentioned above, energy levels in electrons and their interactions determine the chemical behavior and bonding type of materials. Among these cases, the following examples can be mentioned:
- Atoms may produce compounds as a result of ionization .
- Atoms create new compounds by sharing electrons in covalent bonds .

Magnetism and electricity
Static electricity is caused by the movement of electrons from one part to another. This type of charge separation means that a part will have a positive charge and a part will have a negative charge. Electric current is also the flow of electric charges – usually electrons – the movement of these electrons expresses the concept of electrical conductivity.
Electron and quantum physics
In an atom, electrons are bound to the nucleus through electrostatic attraction. According to classical physics, electrons must join the nucleus due to gravity, losing energy. If this view was correct, atoms would have a short life. Therefore, the existence of atoms cannot be explained with the help of classical physics.
In quantum physics, electrons can assume certain levels of energy and cannot combine with protons in the nucleus under normal conditions. Under certain conditions that occur in merging stars, these electrons combine with protons in the nucleus.
Wave-particle duality
Quantum physics stated the basis of “Wave-Particle Duality”, which means that particles can behave like waves and have speed , wavelength , amplitude and frequency , and can also undergo refraction , scattering and reflection. In this regard, there are some equations that we try to express briefly and in simple language:
Dubroy equation and electron waves
The wavelength of a substance with a wave behavior can be calculated using the de Broglie equation, which has the following relationship:
�=h/p
In relation to the above:
- �: Wavelength
- ℎ: Planck’s constant
- �: Momentum _
Schrödinger’s equation
Using the Schrödinger equation , the quantum mechanical wave function of the electronΨcan be solved. to helpΨAndΨ2, we get three of the four quantum numbers to characterize the electrons in the atom or molecule and the shape and direction of the electron orbitals.

Dirac equation and electron spin
The wave equation for the movement of electrons with a speed close to the speed of light describes the spin of the electron, which is called the Dirac equation. Electrons spin as±12and this means that electrons belong to a group of subatomic particles.
Quantum numbers
Each electron in an atom is described by four numbers known as quantum numbers. These numbers are:
- �: principal quantum number
- �: orbital angular momentum quantum number (sub-quantum number)
- ��: Magnetic quantum number
- ��: spin quantum number
Regarding quantum numbers and electrons, the ” Pauli Exclusion Principle ” states that no two electrons in the same atom can have the same four quantum numbers. Therefore, the position of each electron in the atom is a unique position.
Angular momentum
In addition to mass and charge, electrons also have angular momentum . This impulse exists in two ways:
- Angular momentum when the electron rotates around the nucleus
- spin angular momentum
If this article was useful for you, the following tutorials and content are also recommended for you:
- A collection of chemistry lessons
- A collection of physics tutorials
- Elementary physics education 3
- What is antihydrogen? – in a simple word
- Spectroscopy — in simple terms