Coagulants are compounds that cause particle flocculation by neutralizing the charge of suspended particles. These compounds are widely used in water purification processes. Among the types of these materials, mineral salts such as alum and polymer compounds can be mentioned. In the rest of the text, we intend to introduce you to the way coagulants work and their types, so stay with us.
Why is it important to use coagulants?
These materials are among the compounds that are widely used in water purification processes to separate suspended particles and remove excess phosphorus in water. These materials are generally divided into two categories, organic and inorganic. Usually, organic types are used to separate solid and liquid particles and produce sediment. Polyamines and PolyDADMACs can be mentioned among the most important organic compounds. These compounds in the cationic state can neutralize negatively charged suspended particles and create microflocs. Among the advantages of using these materials, we can point out that it does not affect pH, produces less sediment and sludge, and requires less amounts.
Types of coagulants in water treatment
There are different types of coagulants including alum, iron sulfate and cationic polyelectrolytes . All these substances, by reducing the zeta potential of the colloidal system, lead to more collisions of colloidal particles with each other and continue to coagulate by slowly mixing them with each other. Alkaline and acidic aluminum salts are used to clean and clarify water. Trivalent iron salts are much more practical, but its bivalence is also used to remove phosphorus from water. In general, coagulants are divided into two categories:
- organic coagulant (polymeric)
- Mineral coagulant
Organic coagulants
These coagulants are used to separate solid materials in water and produce sludge. There are two types of organic water treatment chemicals. The first is polyamines such as polydiallyldimethylammonium chloride, or polyDADMAC, which are the most widely used. These materials are most effective in treating sewage and very cloudy raw water and work by neutralizing the particle load. The second type of wastewater organic chemicals is melamine formaldehyde and tannin, which are used to coagulate colloidal substances in water. These materials are especially suitable for removing sludge. Because they can absorb organic substances such as oil and fat.
The advantage of polymer coagulant compared to other coagulants such as alum is that polymer coagulants have a very high molecular mass that can easily cause sedimentation.
Structure of polymeric coagulants
The most common anionic groups of coagulant materials in the structure of anionic polyelectrolytes are carboxylate group (-COO-), phosphonate group (-PO3H-, -PO32-) and sulfonate group (-SO3-). In cationic polyelectrolytes, the most common functional groups are amines of the first, second, and fourth types (–NH3+, =NH2+ & ≡N+).
The type of anion present in the structure of polymer coagulants and its repetition in the polymer structure indicate polyelectrolyte properties such as solubility in water and other polar solutions with hydrogen bonding (such as alcohol), electrical conductivity and solution viscosity. Unlike non-ionic polyelectrolytes, these properties strongly depend on pH and salt concentration. All natural or synthetic polyelectrolytes can be produced on a large scale. Natural polyelectrolytes such as pectin, alginate and polypeptides. Examples of synthetic polyelectrolytes are polyallylamines and their salts.
Among the most important polyelectrolytes used in water purification, we can mention polyacrylamide, which we will discuss further:
Polyacrylamide
The term polyacrylamide refers to any polymer that has acrylamide as one of its monomers. If we want to check more carefully about this substance, its Iopak name is (poly (prop-2-enamide), which is a water-soluble polymer and is obtained from the polymerization of acrylamide and N, N′-methylenebis) acrylamide monomers . Come.

Polyacrylamides are swelling polymers, the only commercial type of which is poly(2-propenamide), which is called polyacrylamide. These types of polymers can be made linearly or cross-linked depending on their use.PAMIt increases the viscosity of water and causes the particles in it to coagulate. Cross-linking polymers can absorb a large amount of water due to the presence of amide groups that form hydrogen bonds with water, which is called PAM gel. Molecular weight Commercial polyacrylamides range from 105 to more than 107.
Polyacrylamide is nonionic with acrylamide monomers alone. In order to obtain anionic polyacrylamide, it is necessary to have acrylate or 2-(acrylamido-2-methylpropane sulfonate) monomers (AMPS) with different percentages in the structure of polyacrylamide.
Also, in the structure of coagulants and flocculants of cationic polyacrylamides, instead of acrylamide monomers, other monomers such as:
- dimethyldiallylammonium
- (ethanaminium (N, N, N-trimethyl-2-((1-oxo-2-propenyl) oxy
- 1,2-dimethyl-5-vinylpyridinum
are present
Application of PAM in the environment
In general, polyacrylamide is widely used in environmental fields, including:
1) as a viscosity improver in enhanced oil recovery (EOR) and more recently as a friction reducer in hydraulic fracturing or HVHF
2) as a coagulant and flocculant in water purification and dewatering from sludge
3) as an agent for improving soil properties in agricultural applications
The structural form of different polyacrylamides can be seen in the following table:
| Structure | name | Grouping |
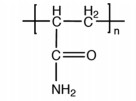 |
Polyacrylamide | Non-ionic polyacrylamide
PAM |
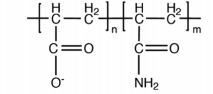 |
Polyacrylamide-co acrylic acid, hydrolyzed polyacrylamide |
Anionic polyacrylamide
WHATEVER |
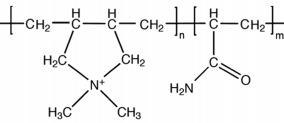 |
poly(AM-co (DADMAC |
Cationic polyacrylamide
CPAM |
Cationic polymer coagulants
Cationic polyacrylamides are also used as emulsion separators and to increase the efficiency of filtration and dewatering from sludge. Like the coagulants, this material also helps to separate solid materials by neutralizing the surface charge of colloidal particles and then settling them, and also reduces the volume of the remaining sludge.
In paper industry wastewater treatment, this material increases the sedimentation rate. Gel electrophoresis for the separation of supermolecules is also one of the other important applications of these materials, which has been less studied than other applications. When an electric current passes through the polyacrylamide gel, proteins or nucleic acids move towards the positive electrode. they do. Since their movement depends on the charge and size of the molecules, each of the molecules moves to a certain direction in the gel matrix. Non-ionic polyacrylamides are also used for mineral applications and textile industries.
One of the applications of polyacrylamide is to use it as a stabilizer in lotions and other cosmetic products. Although this substance itself is not problematic or dangerous, its constituent monomers are highly carcinogenic. The European Union has set a limit on the amount of monoacrylamides for products that are made of polyacrylamide, but the United States of America has not yet set a ban on the use of this substance. This material is used in cosmetic products such as facial moisturizers, anti-aging products, lotions, hair products and sunscreens.
Coagulant polymers
In discussing the use of these coagulant and flocculant materials in water and wastewater treatment, it should be mentioned that the effect of the flocculation process is evaluated based on the reduction of turbidity, TSS, COD, and color, and according to research, these parameters decrease by 90% in optimal conditions. . The very high use of polymers as flocculants is due to their ease of use and no need for constant pH adjustment. On the other hand, very small amounts of them have a great impact in the field of consumption, such as wastewater treatment.
The main characteristics of polymers used as flocculants can be as follows:
| Grouping | characteristic | |
| Amphoteric/anionic/cationic/nonionic | surface charge | |
| 1 to 3 million | less | Molecular Weight |
| 3 to 6 million | medium | |
| 6 to 10 million | Standard | |
| 10 to 15 million | Much | |
| More than 15 million | very much | |
| 1 to 10% | less | surface charge |
| 10 to 40% | medium | |
| 40 to 80% | Much | |
| 80 to 100% | very much | |
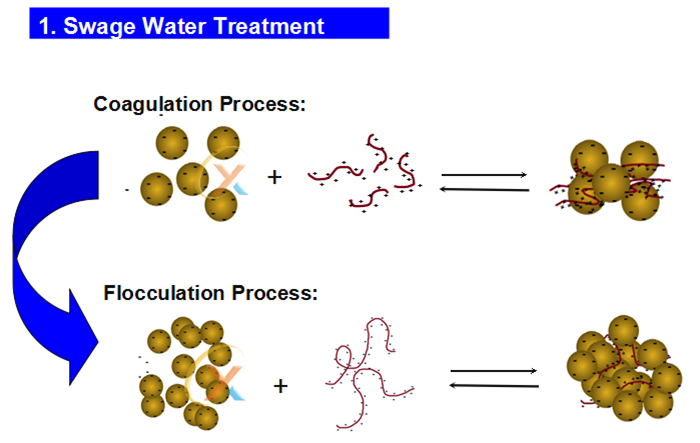
Polyacrylamide has attracted a lot of attention due to its economic and easy application. There is also the ability to synthesize this material as a flocculant in different weights and different charge densities and in three types of anionic, non-ionic and cationic. According to the customer’s needs, the type of surface charge and the amount of molecular weight can be changed. The following figure shows how this substance works in wastewater treatment and bridging between particles.
Mineral coagulants
These materials are usually cheaper and more economical than their organic counterparts and are used in a wide range of purification processes. They are acidic, and for this reason, care must be taken when storing and transporting them. Mineral coagulants are especially effective in treating raw water with low turbidity, and if organic coagulants are insufficient, it can be used to treat this type of water. These materials are generally based on aluminum or iron. Aluminum sulfate is the most common wastewater treatment chemical in the world. Other examples of these materials include aluminum chloride, polyaluminum chloride , iron sulfate, and ferric chloride.
The following are the characteristics of a good coagulant:
- contain sulfate or chloride groups.
- be liquid or solid. Most of them are in aqueous solutions such as aluminum sulfate or ferric chloride.
- be acidic or alkaline. Aluminum salts such as polyaluminum chloride have a certain level of alkalinity.
- have the ability to bind to a number of divalent or trivalent atoms, and Ahem salts can act as divalent salts.
Factors affecting the coagulation process
Many factors affect coagulant performance, among which the following can be mentioned:
- Alkalinity of water
- pH of the environment
- temperature
- Amount of suspended matter in water
- The amount of organic matter in water
Increasing pH, water alkalinity, suspended solids concentration, temperature, as well as reducing organic matter helps to improve the flocculation process.
What is the difference between coagulant and flocculant?
Coagulant means coagulant while flocculant is coagulant. The process of coagulation is a chemical process in which it takes place by dissimilar charges and attraction of dissimilar charges to each other. While flocculation is a physical process and does not involve charge neutralization. The coagulation-flocculation process can be used as a preliminary or intermediate step between other water or wastewater treatment processes such as filtration and sedimentation.
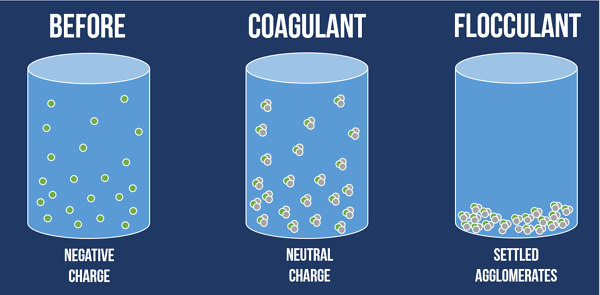
Application of coagulants
In water treatment, coagulants are used to remove a wide range of hazardous substances, including organic substances and pathogens, inorganic and toxic substances, such as arsenic, chemical phosphorus and fluoride from water.
Other uses include the following:
- Medical applications for blood clotting
- paper production
- textiles
- Oil and gas operations
- mines